Minaris designs and develops research, production and platform cell lines that are stable, high-performing and ready to scale. We engineer lines for producing viral vectors and recombinant proteins.
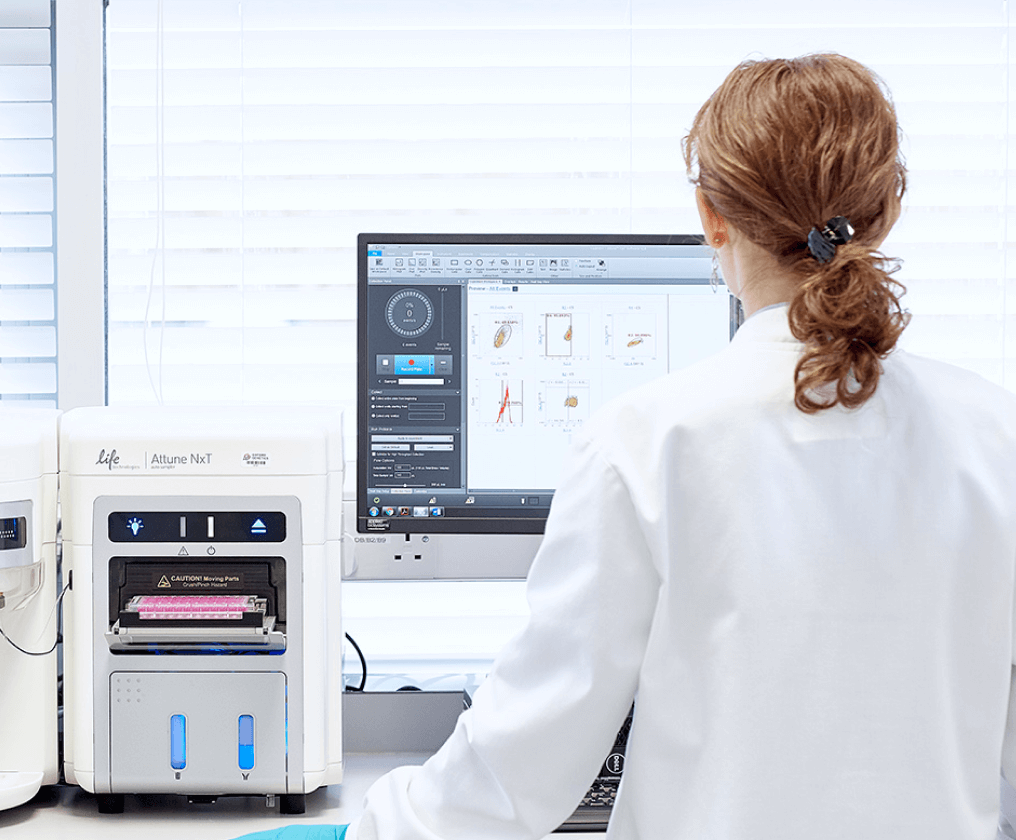
High-throughput clone screening is employed to maximize the chances of obtaining a clone with the desired level of performance. We test such performance using predictive small-scale bioreactors and processes to de-risk the scale-up process. Each selected clone is rigorously characterized to ensure compliance with regulatory guidelines.