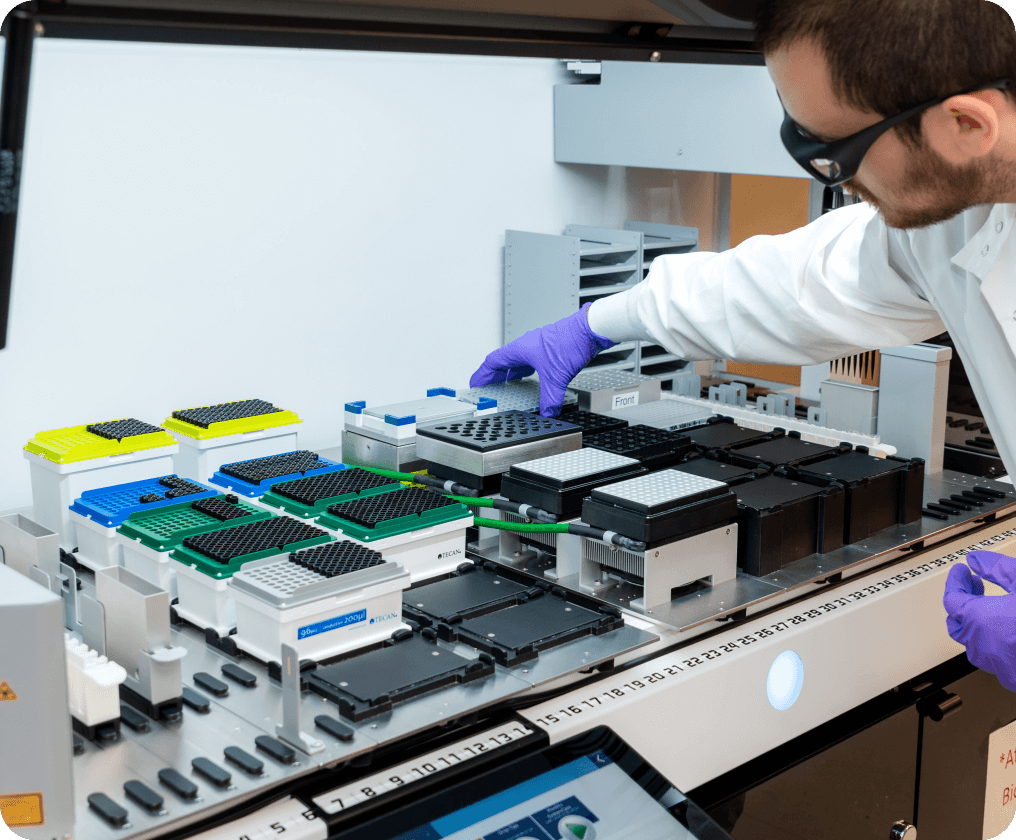
The testing facility located in Philadelphia is the cornerstone of Minaris’ testing network. At 140,000 square feet, it is one of the largest dedicated biosafety and analytical testing buildings in the industry. Conveniently located just 15 minutes from Philadelphia International Airport, the site enhances supply chain efficiency, ensuring rapid sample intake and distribution for global clients.
The site integrates molecular biology, virology, microbiology and quality control laboratories under one roof, enabling seamless coordination across testing disciplines. Expanded in 2021, our testing facility increased sample throughput by more than 130 percent, with significant gains in virology, microbiology and quality control. Today, the site supports over 1,700 developed assays. The flagship of Minaris’ testing network is a purpose-built facility for biosafety and analytical excellence.